Abstract
Background: Autoimmune (AI) disorders, in particular Sjögren's syndrome (SS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE), have been increasingly recognized as risk factors for diffuse large B cell lymphoma (DLBCL). It remains controversial whether increased lymphoma risk arises from the diseases themselves or the immunosuppressive agents used to treat them. It is possible that AI-associated lymphomas comprise a distinct lymphoma subset, exhibiting their own characteristic clinical and biologic behavior. In a recent analysis of >5,000 DLBCL patients in a large national database, our group described decreased lymphoma-related survival in patients with concomitant RA and a trend towards decreased survival in patients with SLE (Koff JL, Clin Lymphoma Myeloma Leuk 2018). Unfortunately, this database did not contain data about cell-of-origin subtype, which is highly associated with survival in DLBCL, or immunosuppressive regimen used to treat AI disease. To determine the prevalence of AI-associated disease among DLBCL patients and better characterize this unique population in terms of demographics, clinical features, and survival outcomes, we examined a large institutional database that included more granular information regarding tumor immunohistochemical (IHC) markers and AI disease treatment.
Methods: We used an existing clinic-based registry of DLBCLs to identify patients diagnosed with DLBCL between 1989 and 2018 with concomitant AI disease as defined by the American College of Rheumatology. To be included, AI disease diagnosis was required to precede diagnosis of DLBCL by a minimum of six months. Patient age, sex, race, dates of DLBCL and AI diagnoses, stage, International Prognostic Index (IPI) score, poor performance status (ECOG score ≥2), B symptoms, lymphoma treatment strategies, date of progression/relapse, and date of death were identified via chart review. We also examined history of immunosuppressive therapies for AI disorders, AI serum markers such as ANA and rheumatoid factor, DLBCL cell-of-origin subtype by IHC Hans algorithm, and "double-hit" status by fluorescent in situ hybridization testing for translocations of MYC, BCL2, and BCL6. First-line treatments were categorized as: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), rituximab-CHOP (R-CHOP), and "other." We analyzed demographics and baseline clinical characteristics for patients with DLBCL and concurrent SS, RA, SLE, or any other AI disease, plotted overall survival (OS) and progression-free survival (PFS) for these groups, and compared median survival times.
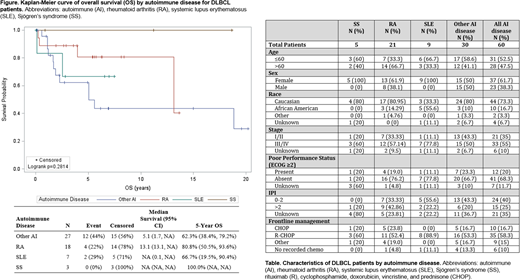
Results: Upon initial chart review of 1,045 total patients with DLBCL, 60 patients had a preceding diagnosis of AI disease; of these, 35 carried a diagnosis of either SS, RA, or SLE. Patient characteristics are summarized in the Table. Compared to DLBCL patients with other AI diseases, patients with SS, RA, or SLE were more commonly female (73.3% vs 44.4%, p=0.026), more likely to experience B symptoms (46.7% vs 18.5%, p=0.033) and more likely to have a history of methotrexate treatment (36.7% vs 11.1%, p=0.037). All patients received similar first-line DLBCL treatments regardless of AI disease type. Data on IHC results and FISH status was inconsistently documented prior to 2010. AI factors of interest, including serum markers and immunosuppressive therapies, were also not reliably recorded for any era. Compared to patients with SS and RA, there was a trend towards lower OS in patients with SLE and other AI diseases, although this difference was not statistically significant (Figure). PFS was not significantly different between any groups (range, 41.9%-55.0%).
Conclusions: In this retrospective study of over 1,000 patients with DLBCL, concomitant AI disease was uncommon. A more complete evaluation of factors impacting survival in AI-associated DLBCL was limited by small sample size and incomplete documentation of key lymphoma and AI disease features in this dataset. The possibility of lower OS for patients with SLE and other AI diseases should be explored in future prospective studies, which may also better capture molecular features of both DLBCL and AI disease.
Flowers:Pharmacyclics/ Janssen: Consultancy; Gilead: Consultancy; Gilead: Research Funding; Abbvie: Research Funding; Burroughs Wellcome Fund: Research Funding; Janssen Pharmaceutical: Research Funding; Genentech/Roche: Research Funding; Spectrum: Consultancy; V Foundation: Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Research Funding; OptumRx: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Karyopharm: Consultancy; Genentech/Roche: Consultancy; National Cancer Institute: Research Funding; Denovo Biopharma: Consultancy; Millennium/Takeda: Research Funding; Bayer: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Research Funding; BeiGene: Research Funding; Acerta: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal